

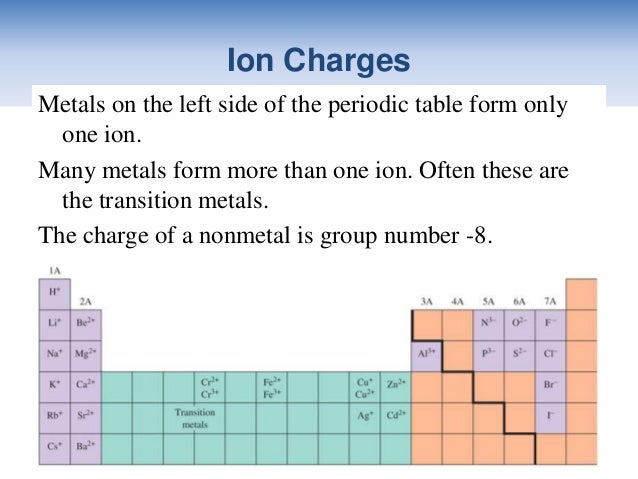
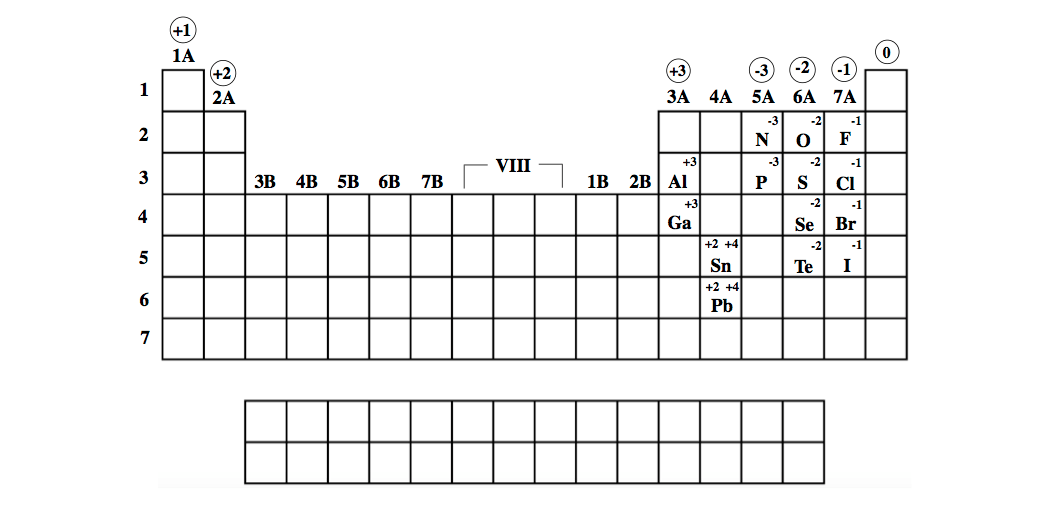
TABLE 6.2 Names and formulas of some common ionic Several ionic compounds are listed in TableĦ.2, with both their common and systematic names. AlthoughĪll compounds have systematic names, many also have trivial, or common, names. Systematic names and are based on a set of rules drawn up by IUPAC. Of the compound and perhaps something of its properties. Ideally, this name should indicate the composition
CRC Handbook of Chemistry and Physics (85th ed.). Half-life, spin, and isomer data selected from the following sources.International Union of Pure and Applied Chemistry. "News & Notices: Standard Atomic Weights Revised"."Atomic weights of the elements 2005 (IUPAC Technical Report)". de Laeter, John Robert Böhlke, John Karl De Bièvre, Paul Hidaka, Hiroshi Peiser, H."The NUBASE2020 evaluation of nuclear properties" (PDF). Isotopic compositions and standard atomic masses from:.
"The AME 2020 atomic mass evaluation (II). ^ Half-life, decay mode, nuclear spin, and isotopic composition is sourced in:."Standard atomic weights of the elements 2021 (IUPAC Technical Report)". ^ Prohaska, Thomas Irrgeher, Johanna Benefield, Jacqueline et al.107Pd versus 107Ag correlations observed in bodies, which have clearly been melted since the accretion of the Solar System, must reflect the presence of live short-lived nuclides in the early Solar System. The discoverers suggest that the coalescence and differentiation of iron-cored small planets may have occurred 10 million years after a nucleosynthetic event. Radiogenic 107Ag was first discovered in the Santa Clara meteorite in 1978. Iron meteorites are the only objects with a high enough palladium/silver ratio to yield measurable variations in 107Ag abundance. The palladium isotope 107Pd decays by beta emission to 107Ag with a half-life of 6.5 million years. The primary decay products before 107Ag are palladium (element 46) isotopes and the primary products after are cadmium (element 48) isotopes. The primary decay mode before the most abundant stable isotope, 107Ag, is electron capture and the primary mode after is beta decay. Isotopes of silver range in atomic weight from 91.960 u ( 92Ag) to 132.969 u ( 133Ag). This element has numerous meta states, with the most stable being 108mAg (half-life 439 years), 110mAg (half-life 249.86 days) and 106mAg (half-life 8.28 days). 40 radioisotopes have been characterized with the most stable being 105Ag with a half-life of 41.29 days, 111Ag with a half-life of 7.43 days, and 112Ag with a half-life of 3.13 hours.Īll of the remaining radioactive isotopes have half-lives that are less than an hour, and the majority of these have half-lives that are less than 3 minutes. Naturally occurring silver ( 47Ag) is composed of the two stable isotopes 107Ag and 109Ag in almost equal proportions, with 107Ag being slightly more abundant (51.839% natural abundance).


 0 kommentar(er)
0 kommentar(er)
